A framework for chemical safety assessment incorporating NAMs within REACH
Current chemical safety assessment
The safety assessment of chemicals is currently based on in vivo data obtained from observational studies. These in vivo studies allow to determine safe limit doses (e.g. acceptable operator exposure levels and acceptable daily intake), characterize potential harm and classify hazards. However, the use of animals raises ethical, economical, and scientific concerns.
Therefore, in recent years, a lot of research has been conducted in the field of New Approach Methodologies (NAMs), with the development of new in vitro and in silico tools. These new methods and models can be used to partially replace experimental animals. However, at the moment, a full replacement of in vivo tests can only be achieved for straightforward local toxicity endpoints (e.g. skin and eye irritation). A full replacement remains quite challenging for more complex endpoints including systemic toxicity. Consequently, the use of NAMs is poorly considered in the current regulatory framework for chemical safety assessment. Indeed, most of the recently developed models are not yet sufficiently validated which entails that no safety decisions can be made based on NAMs.
Tiered Approach as a new framework
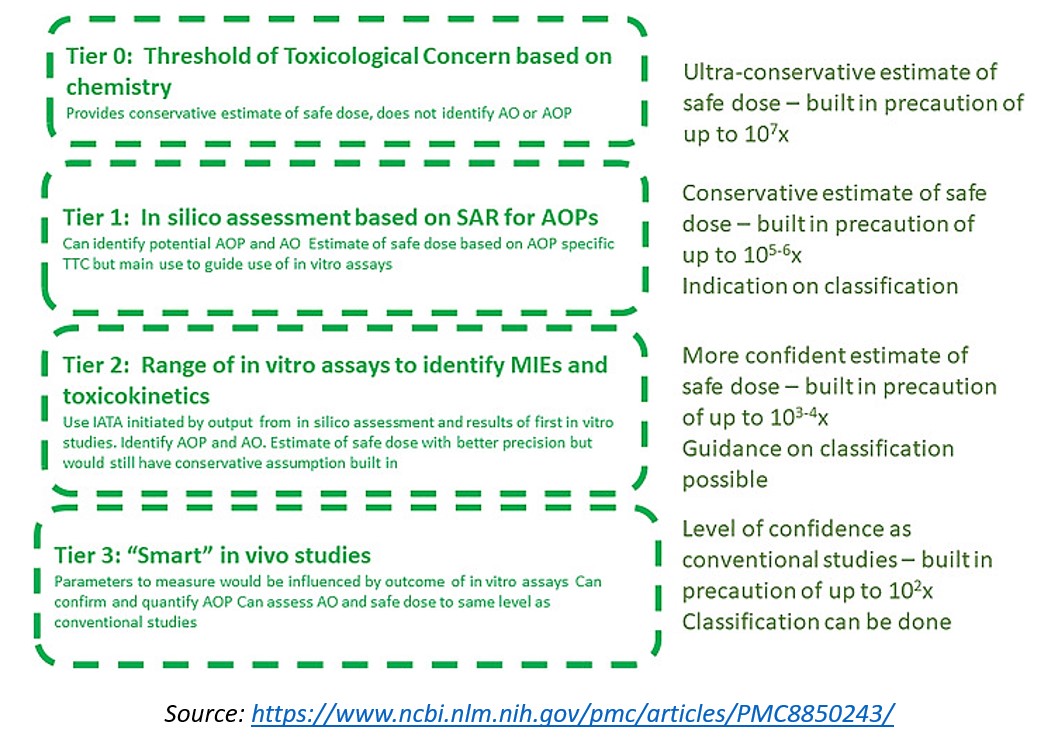
Observational studies have provided a wealth of data, and it is now possible to predict adverse outcomes based on the affected biological pathways and the chemical structure of a compound. Consequently, experts from the European Centre for Ecotoxicology and Toxicity of chemicals propose a Tiered Approach for safety assessment of chemicals based on the adverse outcome prediction which meets the requirement of the REACH legislation.
The Tiered Approach assumes that adverse outcome predictions can be made with greater precision via the use of NAMs. The approach would go through in silico (tier 1) to in vitro (tier 2) to in vivo (tier 3). The probability of hazard, the category and the safe dose profile would then be evaluated at each step, depending on the output from the previous tier and providing results with more certainty. The in vivo tier 3 studies will only be used as a last step to determine if the hazard potency seen in vitro will be observed in vivo and to see if important adverse effects have been missed during previous steps.
Moreover, the implementation of NAMs in safety assessment will depend on their level of confidence. For skin and eye corrosion, mutagenicity, dermal sensitisation, and acute toxicity, NAMs can already be used with a high level of confidence. However, for repeated dose toxicity, reproduction toxicity and developmental toxicity, the new assays are only in development and cannot completely replace in vivo experiments.
Stepwise implementation of NAMs
Today, a full replacement of animal testing by NAMs for chemical risk assessment is quite unrealistic. NAMs should be gradually introduced, building more confidence with the new technologies and should be driven by regulatory agencies in consultation with relevant stakeholders. This will help to make safety decisions with fewer animals.
Sources:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8850243/